FDA 510(K) Stats Analysis
Customer Challenges
- Review of regulator pre-submission responses and test protocols in relation to statistical methodology and sample size, with assessment on methodology and sample size choice.
- Advise on strategy for ongoing change control activities in relation to data output and testing confidence intervals.
- Design and development of an efficient and statistically significant product test protocol by accounting for samples of all test variables.
- Statistical analysis of clinical and non-clinical test results with documentation of the results of the statistical review for inclusion in regulatory submission.
Solution
Detailed statistical comparative analysis of client medical device against published results from compative device in the market. Statistical approaches included:
Data Visualisation - Boxplots / Histograms - Outlier Analysis
Analysis of Variance (ANOVA) - Normality Tests (Wilks, etc.)
Comparisons of means - Comparisons of variances with Levene’s test on equivalency
Comparisons of means - Comparisons of variances with Levene’s test on equivalency
Repeated measures ANOVA - Kruskal Wallis tests
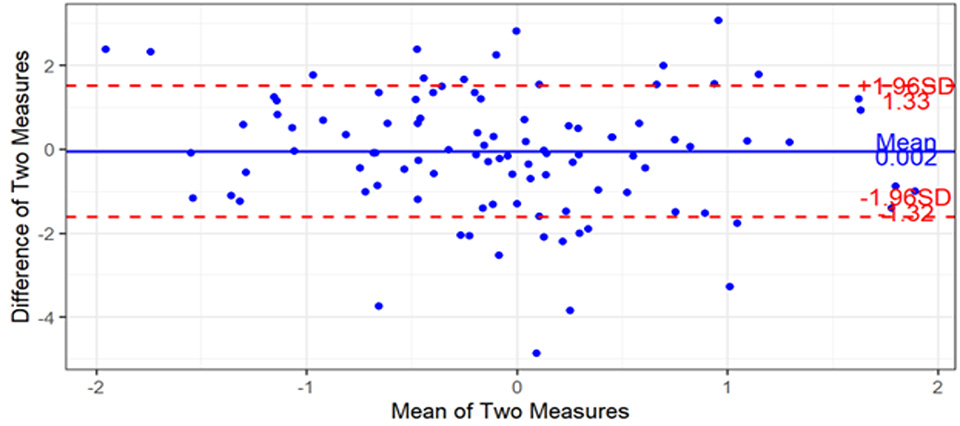
Difference Charts - Bland Altman analysis
The statistical results and analysis were presented to the client in a detailed report that was included in the FDA 510(K) market entry application.
Benefits
Clarke Analytics participated in a call with the FDA to discuss aspects of the analysis. This facilitated the FDA accepting the analysis with greater confidence in statistical analysis rigour with separation from sponsoring organisation.
Related Case Studies
Medical Devices
A medical device company was experiencing increased levels of scraped product in their manufacturing plant.
Public Sector
Analytics Innovation Workshop to work through key KPI’s of interest;
Analytics Innovation Lab with collaborate supply chain analytics partner.
Retail
Over a 3 month period, we worked with the client to understand their key business questions. This began with an innovation workshop with key business and IT stakeholders, followed by a structured analysis of muitiple datasets.
Let's explore the art of the possible